High-entropy oxides (HEOs) represent an emerging class of ceramic materials characterized by the incorporation of five or more principal metal cations in near-equimolar proportions within a single crystalline phase. This configurational disorder, driven by high entropy contributions, enables the stabilization of structures that would otherwise be thermodynamically unstable in conventional oxide systems.
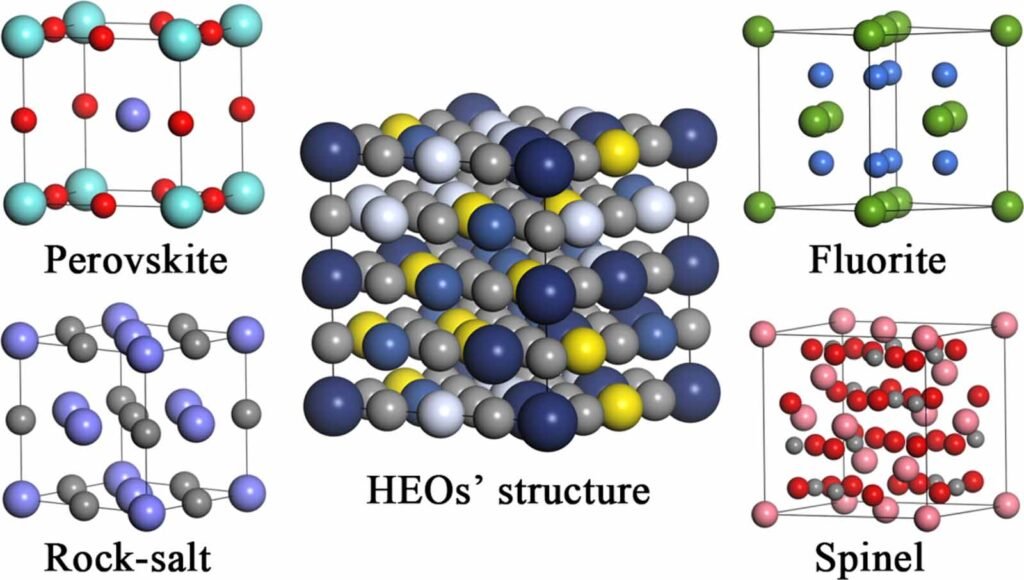
(Image taken from Zhong Yang et al 2024 Mater. Futures 3 042103)
A significant contribution to this field was published in Nature Communications, under the title “Thermodynamics-inspired high-entropy oxide synthesis” by Saeed S. I. Almishal and colleagues from The Pennsylvania State University and collaborators (Almishal et al., Nat. Commun. 16, 8211; 2025). The study demonstrates that oxygen chemical potential—controlled via oxygen partial pressure (pO₂) during synthesis—serves as a critical and previously underutilized thermodynamic variable for achieving phase-pure rock salt–structured HEOs, particularly those incorporating multivalent cations such as manganese (Mn) and iron (Fe).
Challenges in Conventional HEO Synthesis
In rock salt HEOs, such as the MgCoNiCuZnO system, all cations ideally adopt a divalent (+2) oxidation state to maintain structural compatibility and charge neutrality. However, multivalent elements like Mn and Fe exhibit a strong tendency to form higher oxidation states (+3 or higher) under ambient oxygen conditions (pO₂ ≈ 0.21 atm), leading to phase segregation into secondary phases such as spinels or sesquioxides. This has historically limited the compositional diversity of stable rock salt HEOs.
The Role of Oxygen Chemical Potential
The authors propose that HEO thermodynamics extend beyond temperature-driven entropy stabilization to encompass a multidimensional phase space, with oxygen chemical potential (μO) as a decisive axis. By conducting synthesis under reduced pO₂ environments (typically 10⁻⁶ to 10⁻⁸ atm, achieved via argon gas flow), the equilibrium is shifted to favor the divalent states of Mn and Fe.
To guide composition selection and predict synthesizability, the following tools were developed:
- Preferred valence phase diagrams derived from CALPHAD thermodynamic assessments, mapping stable oxidation states as functions of temperature and pO₂.
- High-throughput enthalpic stability maps employing machine-learning interatomic potentials (e.g., CHGNet), which correlate mixing enthalpy (ΔHmix) and bond-length variance (σbonds) to identify low-energy compositions.
- Oxygen chemical potential overlap (μoverlap), a novel descriptor quantifying the overlap in μO stability windows for divalent states across all constituent cations. Positive μoverlap indicates a feasible synthesis regime under accessible conditions.
Using this framework, the team identified and successfully synthesized seven equimolar, single-phase rock salt HEOs incorporating Mn and/or Fe, including five-component systems such as (MgCoNiZnMn)O and (MgCoNiMnFe)O, as well as a six-component variant (MgCoNiZnMnFe)O.
Outlook
This work establishes oxygen chemical potential as a practical and predictive design parameter, enabling the incorporation of abundant, multivalent elements into HEOs without exotic precursors or extreme processing conditions. The resulting materials expand the palette of accessible compositions for applications in catalysis, energy storage, dielectrics, and magnetic systems.
The proposed thermodynamic framework is extensible to other oxide structures (e.g., fluorite, perovskite) and multivalent chemistries, potentially accelerating the discovery of next-generation functional ceramics. Future investigations may explore the magnetic, electronic, and catalytic properties of these newly stabilized HEOs, as well as adaptations for thin-film or additive manufacturing routes.





